Blog
Nelson curve.
HTHA: Refineries' Major Concern
HTHA, or High Temperature Hydrogen Attack, describes the cracking degradation suffered by certain types of steel operating under high-temperature conditions (above 400°C) in hydrogen-rich environments. Typically, these conditions occur in equipment found in refineries, petrochemical plants, and other chemical boiler facilities. This type of hydrogen-induced embrittlement behaves differently from that induced during the manufacturing or welding process and is much less studied. HTHA results from the dissociation and dissolution of hydrogen into the solid crystalline network of the steel and its reaction with the carbon of the material under certain circumstances, forming methane and promoting crack formation. This phenomenon can lead to surface decarburization in steel when the reaction mainly occurs on the material’s surface. Internal decarburization can also occur when atomic hydrogen penetrates the material, reacts with carbon producing methane, and accumulates at grain boundaries and/or existing precipitates between the steel’s phases. This effect is particularly dangerous when hydrogen cannot diffuse out of the material and continues to cause typical HTHA cracking and fissures.
The loss of carbon on the steel’s surface results in localized hardness loss. However, internal decarburization and particularly methane formation subsequently lead to the formation of internal voids and can result in substantial deterioration of mechanical properties and eventual catastrophic failures.
The main factors influencing HTHA are hydrogen partial pressure, steel temperature, and exposure time. Hydrogen damage usually occurs after an incubation period that can vary from several hours to many years depending on the severity of the environment. High-temperature and low hydrogen partial pressure conditions favor surface decarburization, while low temperatures and high hydrogen pressure favor internal cracking.
Additionally, steel composition has a significant influence on HTHA resistance, particularly elements that stabilize carbon in the steel structure, making their carbides stable. Elements such as Cr, Mo, and vanadium are particularly important in designing steels less susceptible to HTHA.
The Nelson curve has gaps in the study of hydrogen attack.
Since 1949, NELSON analyzed a series of experimental observations on steels operating at high hydrogen pressure and high temperature, designing the well-known Nelson curves or diagrams. The Nelson curves represent the relationship between temperature and hydrogen partial pressure on a graph. Certain limited areas with dashed lines representing “safe operating” zones for structural carbon steels, 1.25Cr-0.5Mo steels, 2.25Cr-1Mo steels, and other high-temperature-resistant steels are depicted on the graph. The safe operating zones have been redefined over the years by including points or working conditions for various materials that have led to catastrophic failures in the industry. This diagram has been edited several times by the American Petroleum Institute (API) and included in its recommended practice API 941.
Recent studies, including confidential ones, have been published regarding a catastrophe in Washington in 2010. Specifically, we refer to the May 2014 study on failure analysis conducted by the U.S. Chemical Safety and Hazard Investigation Board regarding the catastrophe that occurred in April 2010 at the Anacortes Refinery, Washington, owned by Tesoro Refining and Marketing Company LLC. At that plant, a catastrophic rupture of a heat exchanger occurred in the Naphtha hydrotreating unit, unit NHT. According to the mentioned report, the catastrophe, which ultimately caused 7 deaths and significant material damage, occurred due to high-temperature hydrogen attack, considered the most serious safety incident in the U.S. oil industry since the previous incident at the BP refinery in Texas City in March 2005. Several conclusions from this study point to inadequate equipment calculation using Nelson curves, without considering aspects such as the deterioration of refractory parts of the equipment, which could have operated for a certain time under conditions exceeding those established by its Nelson curve for design. As a result, some time before the final rupture, internal cracks began to occur, ultimately leading to collapse.
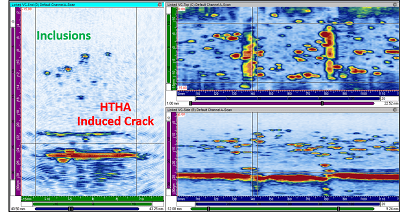
HTHA inspection of metallic material by NDTs.

Anacordes Refinery disaster.
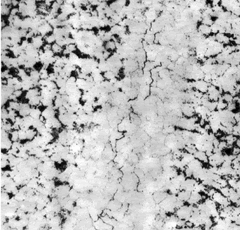
HTHA micrographic test. Lack of C in vicinity of crack.
The sector of major oil companies and refineries, including BP, SHELL, Petrobras, among others, has begun to take an interest in predicting such hydrogen-related failures through periodic inspections. Current ultrasound techniques, however, are severely limited for this application due to the complexity of determining and quantifying hydrogen-induced cracking defects. Specifically, it would be useful to detect them in their early stages, when the first fissures begin to appear.
IDTECHSERVICES has studied the possibility of inducing hydrogen-induced defects of germinal size at high temperatures, simulating conditions above the Nelson curves in various welded materials and base materials for refinery service applications. After several years of studies and tests in hydrogen-rich atmospheres, this is now possible. Materials induced with hydrogen-induced germinal cracks can already be used as calibration standards for non-destructive testing subsequently applied to different equipment, aiming to conduct a comprehensive analysis of the present state of hydrogen damage in some equipment or even develop new inspection procedures for this technique.
IDTECHSERVICES has its own hydrogen reactor for conducting high-pressure and high-temperature hydrogen embrittlement tests on different materials, low-alloy steels for industry, stainless steels for green hydrogen facilities, and aluminum alloys for hydrogen engines.
Fusion line cracks in CrMo reactors with stainless steel overlay. Concerns of the petrochemical industry.
Pressure vessels used in refining/petrochemical processes operate at high temperatures and pressures (e.g., high partial pressures of hydrogen). They are typically constructed from a thick steel wall (with a range that varies widely but is typically between 80 and 350 mm) and can be made from forged shells or curved plates welded longitudinally together. The inner diameter of these structures is typically between 4 and 5 meters, but can reach up to 12 meters, so these pressure vessels can have a total weight ranging from 500 to 800 tons. In some exceptional cases, weights of up to 1400 tons can even be reached. These vessels are subjected to very severe service conditions that can dramatically affect the mechanical and metallurgical properties of the materials they are made of.
Generally, the wall of such a reactor consists of two different materials: a main component of high-temperature-resistant low-alloy steel (structural carbon steel or generally chromium-molybdenum or chromium-molybdenum-vanadium alloys) ensuring the mechanical properties and structural safety of the reactor. Additionally, a thin layer of austenitic stainless steel is applied to the interior zone of the vessel to improve corrosion resistance. In these large reactors, small components may be made, as appropriate, from plated material; however, in thick-walled components, austenitic stainless protection is obtained by weld overlay.

Hydrocracker in refinery.

Disbonding device.
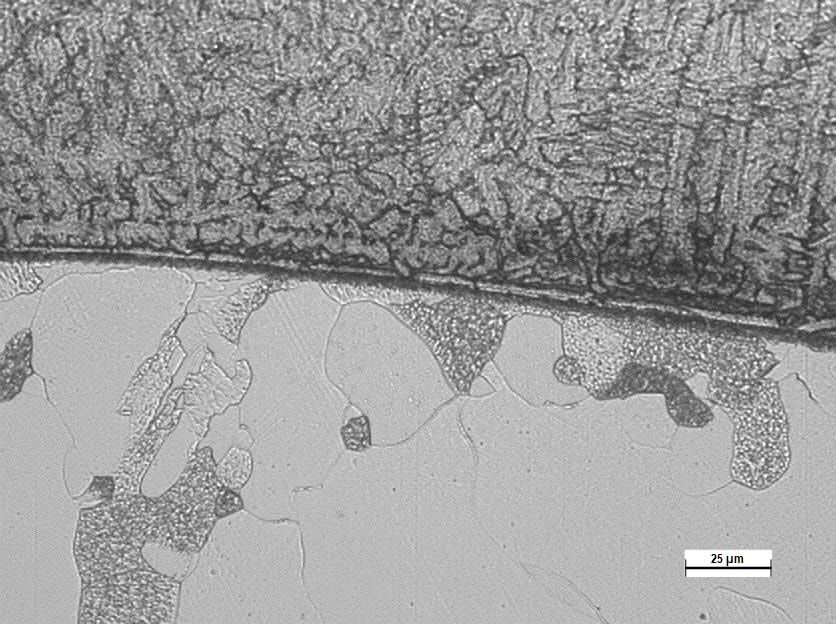
Austeno-ferritic Interfase
Among the possible failures or ruptures present in these reactors, the phenomenon of temper embrittlement is known to require an extended time to manifest. For this reason, only older reactors, such as those that have been in operation for many years at temperatures between 350 and 550°C, may be affected by this type of problem. Due to significant advances made in recent years in reducing impurities in structural steels, the materials currently available have almost ceased to exhibit sensitivity to these types of phenomena.
On the contrary to what happens with temper embrittlement, hydrogen embrittlement can occur within just a few days of service. The risk of brittle fracture caused by hydrogen embrittlement can be noticeable from the first cooling of the reactor during its operating state.
Although the phenomenon of temper embrittlement has been extensively studied, comparatively, only a minority of studies related to hydrogen embrittlement, disbonding, have been conducted. However, this is one of the most critical problems in refinery plants, and understanding it is of vital importance for the industry of oil and gas.
Hydrogen-Induced Disbonding (HID) is defined as a specific degradation induced by hydrogen observed in hydrocracking, hydrodesulfurization, hydrotreating, and hydrogenation reactors, among others, in the petroleum industry. These equipment have the two-component wall structure mentioned. Chromium-Molybdenum steels have been widely used in these refinery applications over the past 50 years and have shown proven ability to withstand severe service conditions such as high temperatures (ranging from 250 to 550°C) and high partial hydrogen pressure (from 30 bar to 230 bar). Both temperature and hydrogen presence have a significant impact on the mechanical stability and microstructure of the base material.
Disbonding consists of the detachment of the interface between the stainless steel overlay and the base metal of the chromium-molybdenum steel, which together constitute the walls of the mentioned reactors.
These equipment operate in hydrogen-rich atmospheres at high temperatures to obtain light hydrocarbon fractions from petroleum. Hydrogen appears in the reactor during the cracking process of heavy hydrocarbon molecules, and it can diffuse into the steel crystallographic network easily due to its small atomic size. In steady-state conditions, an equilibrium is reached between the hydrogen partial pressure inside the reactor and the amount of hydrogen diluted in the reactor wall. During an emergency shutdown, however, this equilibrium is disrupted, and the hydrogen content attempts to reach equilibrium at ambient temperature, enriching the austeno-ferritic interface between the two components with hydrogen. As a result, many problems can arise and occur during the life of the reactors or during their shutdowns and restarts. Specific ruptures can lead to reduced equipment life, and therefore, the interior stainless steel overlaying processes on chromium-molybdenum steel must be validated by tests simulating working conditions and emergency shutdowns.
The occurrence of this phenomenon depends on several factors partially evaluated by other authors, such as:
- Filler metal thickness.
- Base metal thickness.
- Testing temperature and pressure.
- Welding procedure parameters for the stainless steel overlay.
- Post-weld heat treatments.
- Cooling rate simulating emergency shutdown.
- Interface geometry between the overlay and base metal.
IDTECHSERVICES has its own hydrogen reactor to perform hydrogen embrittlement tests according to ASTM G 146, the test standard that regulates the quality of overlays against their resistance to hydrogen attack
